
When the FDA shows up at your generic drug manufacturing facility, it’s not a surprise visit-it’s a verification. Every facility making generic medicines for the U.S. market must pass this test. It’s not about catching mistakes. It’s about proving you’ve built a system that never lets mistakes happen in the first place.
What Happens Before the FDA Arrives
The FDA doesn’t pick facilities randomly. They use a risk-based model that looks at past inspection results, product type, complaint history, and even tips from whistleblowers. If you’ve made the same error twice, or if your product treats a life-threatening condition, your odds of getting inspected go up. That’s why some facilities get checked every year, while others go three or four years between visits.Before any inspection, your facility must be registered with the FDA and your products listed. This isn’t paperwork for the file cabinet-it’s your legal obligation. If you’re applying to sell a new generic drug, you’ll face a Pre-Approval Inspection (PAI). This is different from routine checks. The FDA wants to know three things: Can you make this drug exactly as described in your application? Are your lab methods accurate? And are your stability samples stored under the right conditions?
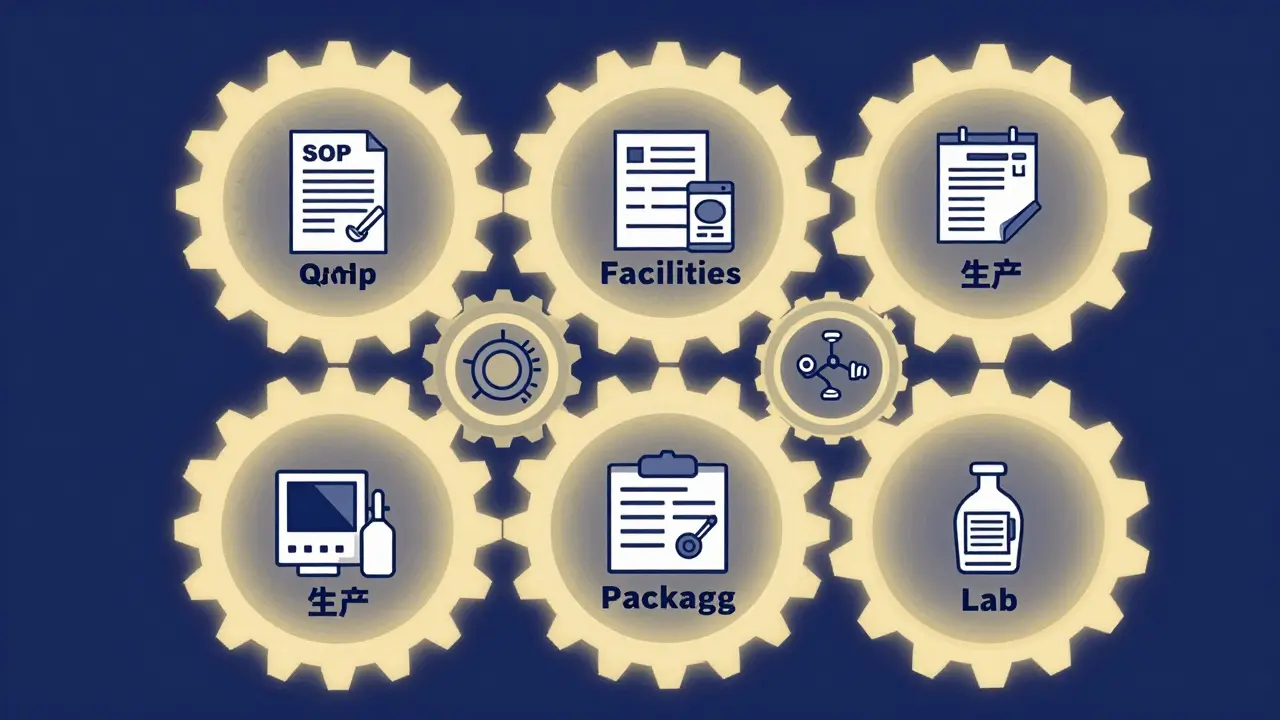
The Six-System Inspection Approach
FDA investigators don’t just walk around. They follow a strict, science-backed framework called the six-system approach. Every inspection covers the Quality System-always. Then they pick two or three others to dig into:- Quality System: This is your backbone. It includes your quality unit, change control, deviation management, and CAPA processes. The FDA checks 21 CFR 211.22(a) to make sure your quality unit has real authority-not just a title on an org chart.
- Facilities & Equipment: Are your clean rooms maintained? Are your equipment logs complete? Is calibration up to date? They’ll ask to see qualification records for every piece of equipment used in production.
- Materials: Where do your raw materials come from? Are suppliers qualified? Do you test incoming batches? If you’re using a new supplier, they’ll want documentation proving it meets the same standards as your original source.
- Production: Can you prove your process is validated? Can you show batch records that match your approved procedures? If you changed a mixing time last month without updating your SOP, that’s a red flag.
- Packaging & Labeling: One wrong label can lead to a recall. They’ll check your label reconciliation, barcode scanning, and how you handle returned or damaged packaging.
- Laboratory Control: Your lab isn’t just for testing finished products. They’ll look at how you validate methods, store reference standards, and handle out-of-spec results. Data integrity is a huge focus now-no backdating, no deleting files, no selective reporting.
It’s not enough to have documents. You need to show they’re alive. The FDA looks for evidence that your systems are actually working-day after day, shift after shift.
What You’ll See During the Inspection
The inspection team usually arrives unannounced. They’ll ask to see your quality unit, your lab, your production floor, and your warehouse. They’ll shadow your staff. They’ll ask operators, supervisors, and even janitors questions. If your quality manager says one thing and a line operator says another, that’s a problem.They’ll request records-lots of them. Batch records, validation reports, equipment logs, training files, deviation reports, and stability data. They’ll compare what’s in your application to what’s in your facility. If your application says you use a 500L reactor, but you’ve upgraded to a 1000L without updating the filing, that’s a violation.
They’ll check your stability chambers. Are the temperature logs accurate? Are samples stored in the right containers? Are you testing at the right intervals? The FDA has caught companies storing stability samples in the wrong room-then claiming they met specs.
They’ll also look at your data systems. Are electronic records backed up? Are access controls in place? Can you prove who made a change and when? If you’re using Excel sheets for critical data, you’re already behind.
The FDA 483 and What Comes Next
If they find issues, they’ll give you Form FDA 483. It’s not a citation. It’s a list of observations. Each item references a specific regulation-like 21 CFR 211.22(a) for a weak quality unit, or 211.160(b) for poor record retention.You have 15 business days to respond. Your response isn’t just an apology. It’s a plan. You need to explain:
- What happened
- Why it happened
- How you’re fixing it
- How you’re preventing it from happening again
Generic responses like “we’ve retrained staff” won’t cut it. The FDA wants proof: updated SOPs, training records, system upgrades, validation reports. They’ll look at your CAPA system to see if you’ve fixed the root cause-or just the symptom.
If your response is weak or incomplete, the FDA may issue a warning letter. That’s a public document. It can delay approvals, trigger more inspections, and scare off partners. But there’s a path forward. In June 2025, the FDA finalized guidance for Post-Warning Letter Meetings (PWLMs). These are structured discussions where you can ask for feedback on your corrective actions before the next inspection.

How to Be Ready-All the Time
Waiting until the FDA knocks is a mistake. The best facilities operate in a permanent state of readiness. That means:- Running mock inspections every quarter with internal auditors
- Keeping SOPs simple, current, and followed by everyone
- Training staff not just on procedures, but on why they matter
- Using digital systems for records-paper is a liability
- Investing in quality culture, not just compliance
The FDA’s PreCheck program, launched in 2024, helps companies avoid surprises. If you’re building a new facility or scaling up, you can submit a Type V Drug Master File (DMF) early. The FDA reviews your design, layout, and quality systems before you start production. It’s not mandatory-but it saves months of delays later.
Companies with mature quality systems don’t just pass inspections. They build trust. The FDA sees them as partners, not problems. That means fewer surprise visits, faster approvals, and more flexibility when changes are needed.
What Success Looks Like
More than 90% of inspections result in an “acceptable” rating. That’s not luck. It’s discipline. The companies that pass consistently don’t have perfect records-they have systems that catch errors before they become violations.One manufacturer in Ohio had 12 consecutive clean inspections. Their secret? Every new employee spends their first week shadowing the quality unit. They don’t learn SOPs from a manual-they learn from the people who live them.
Another facility in Indiana reduced its deviation rate by 70% in a year by making it easy for workers to report issues-no blame, no punishment, just solutions.
The FDA doesn’t want to shut you down. They want you to make safe, reliable medicine. If your facility is built on real quality-not just paperwork-you’ll not only survive the inspection. You’ll thrive because of it.
Stephen Craig
The real test isn't whether you pass the inspection-it's whether your quality system still works the next day when no one's watching.
Most companies treat compliance like a checkbox. The good ones treat it like a habit.
Connor Hale
I've seen facilities with perfect documentation and broken equipment. The FDA knows the difference. Paper doesn't make medicine. People do.
bob bob
My cousin works at a generic plant in Ohio and they do mock inspections every quarter. Everyone knows the drill-no panic, no hiding stuff. Just clean, calm, professional.
They’ve had 11 clean inspections in a row. No magic, just consistency.
Abhishek Mondal
Let’s be honest: the FDA’s six-system approach is a bureaucratic theater. They don’t care about quality-they care about control. The entire system is designed to intimidate small players so Big Pharma can dominate.
And don’t get me started on the 483 forms-half the observations are trivial, the other half are misinterpretations of ambiguous regulations. You’re being punished for not reading their mind.
Terri Gladden
OMG I just read this and I’m crying 😭 like who even has time for all this?? I work in a lab and we’re barely keeping up with the emails, let alone stability chambers and label reconciliation??
Also, why is everyone so obsessed with Excel?? I use it for everything and it’s fine??
Jennifer Glass
There’s something powerful about the idea that safety isn’t a goal-it’s a practice.
The Ohio facility where new hires shadow the quality unit? That’s not training. That’s cultural transmission.
When people understand why a step matters-not just how to do it-they stop cutting corners. Not because they’re scared of the FDA. Because they care.
Joseph Snow
This entire article is corporate propaganda. The FDA isn’t protecting patients-they’re protecting their budget and political capital. They’ve been caught falsifying inspection data. They target small manufacturers because they can’t afford lawyers. Meanwhile, the big pharma giants get waivers and deferred inspections. This isn’t regulation. It’s extortion dressed in lab coats.
melissa cucic
It’s not about avoiding citations-it’s about cultivating a mindset where deviations are treated as data, not failures.
When your CAPA system is truly alive, you’re not reacting-you’re anticipating.
The most resilient facilities don’t have zero errors; they have zero unaddressed patterns.
And yes, digital records aren’t optional anymore-paper is a liability, and anyone still clinging to it is one audit away from disaster.
Also, training isn’t a one-time event-it’s a rhythm, not a checkbox.
And if your quality unit doesn’t have real authority, you’re building a house on sand.
The PreCheck program? Brilliant. Why wait for disaster to prove you’re ready?
Trust is earned through transparency, not perfection.
And yes, I’ve seen this work-multiple times.
It’s not theory. It’s practice.
And it’s the only way forward.
Jacob Milano
I love how this post doesn’t just list rules-it paints a picture of what real quality looks like.
It’s not about the checklist. It’s about the quiet confidence of a team that knows their work matters.
The janitor who knows why the clean room protocol matters? The line operator who speaks up when something feels off? That’s the magic.
That’s the difference between a facility that survives inspection… and one that thrives because of it.
It’s not about fear. It’s about pride.
saurabh singh
From India to the US-this is the same story everywhere.
Quality isn’t a Western idea. It’s a human one.
We had a plant in Pune that went from 80% deviation rate to 10% in 8 months-just by letting workers suggest fixes without fear.
One guy noticed the vial caps were cracking because the machine was too loud-he fixed it with a rubber washer and $2 worth of parts.
No one asked him to do it. He just did.
That’s the culture the FDA wants. Not paperwork. People who care.
Allen Ye
The entire regulatory architecture of pharmaceutical manufacturing is predicated on a fundamental contradiction: the assumption that human systems can be rendered infallible through procedural rigor, when in fact, all systems are inherently fallible by virtue of their human composition.
The FDA’s six-system framework, while ostensibly scientific, functions as a performative ritual of accountability, designed less to ensure safety and more to create the illusion of control.
The obsession with documentation-particularly the demonization of Excel-is not a technical necessity but a cultural artifact of bureaucratic inertia.
Electronic records are not inherently superior; they are merely more traceable-and traceability is not synonymous with quality.
The real metric of success is not inspection outcomes, but patient outcomes-and yet, the FDA has no systematic mechanism to correlate inspection results with post-market safety data.
Furthermore, the PreCheck program, while laudable in intent, merely shifts the burden of proof upstream without addressing the systemic disincentives for innovation in generic manufacturing-namely, the razor-thin margins and the absence of reward for quality beyond compliance.
Until the regulatory framework begins to incentivize excellence rather than penalize failure, we are merely rearranging deck chairs on the Titanic.
The Ohio facility’s success is not a model-it is an outlier.
And outliers are not scalable.
What we need is not more inspections.
What we need is a new paradigm.
mark etang
It is imperative that all entities engaged in the manufacture of generic pharmaceutical products recognize that adherence to the Code of Federal Regulations, specifically Title 21, Chapter I, Subchapter A, Part 211, is not discretionary, but obligatory under the authority of the Federal Food, Drug, and Cosmetic Act, as amended.
Non-compliance constitutes a direct threat to public health and constitutes grounds for regulatory action, including but not limited to Warning Letters, Import Alerts, and Debarment Proceedings.
Therefore, the implementation of robust quality systems, including but not limited to Change Control, Deviation Management, and CAPA, is not merely recommended-it is non-negotiable.
It is further recommended that all electronic records be maintained in accordance with 21 CFR Part 11, and that all personnel receive documented training in accordance with 21 CFR 211.25.
Failure to comply may result in catastrophic consequences for both patient safety and corporate viability.
It is the solemn duty of all stakeholders to uphold the highest standards of integrity, accountability, and scientific rigor at all times.
jigisha Patel
Let’s be real-90% ‘acceptable’ rating? That’s a lie. The FDA lets 90% of facilities slide because they’re overwhelmed and underfunded. The real problem is that 40% of those ‘clean’ inspections were done by interns with 3 weeks of training.
And don’t even get me started on the fact that the same companies that pass inspection are the ones getting hit with recalls six months later.
They’re gaming the system. The FDA knows it. But they don’t have the resources to catch them.
So they scare the small ones to make themselves look tough.
It’s not quality. It’s theater.




Write a comment