
Every year, over 100 million drug shipments enter the United States. Most come from overseas manufacturers in countries like India, China, Germany, and Ireland. The FDA doesn’t inspect every single one. But it doesn’t need to. Instead, it uses a smart, risk-based system to catch the dangerous ones before they reach pharmacy shelves or hospital bins.
What Gets Stopped at the Border?
The FDA doesn’t just check for fake pills or expired medicine. It looks for anything that breaks U.S. standards: drugs made in unregistered factories, products with wrong labels, contaminated ingredients, or shipments that don’t match their paperwork. In 2022, about 14.3% of drug shipments that went through physical inspection were detained. Of those, nearly 68% were eventually refused entry. That’s more than 1 in 10 shipments blocked because they didn’t meet the bar.What gets flagged? Common issues include missing or incorrect product codes, unregistered manufacturing sites, or labeling that doesn’t follow FDA rules. One common mistake? A pill bottle labeled as “ibuprofen” but containing a different active ingredient. That’s not just a typo-it’s a health risk.
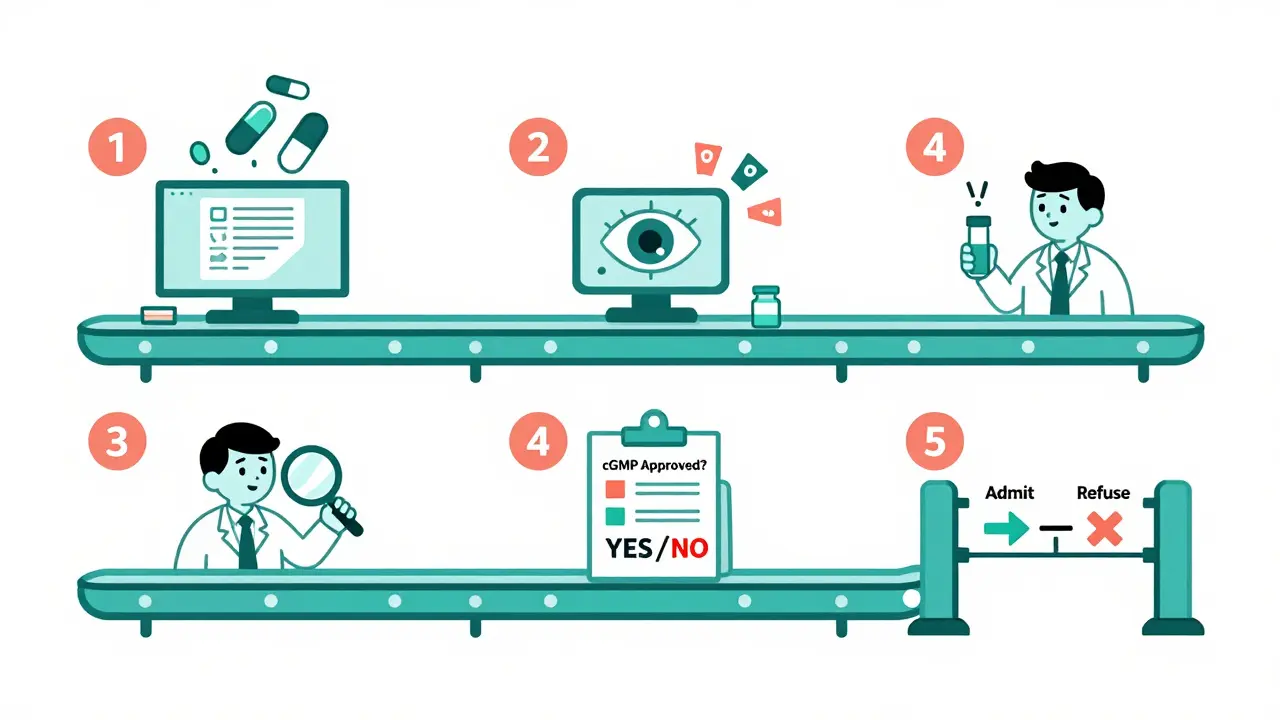
The Five-Step Inspection Process
The FDA’s import system runs like a well-oiled machine, even if it’s not always fast. Here’s how it works:- Entry Submission: Importers file electronic paperwork through the FDA’s system. This includes the product name, quantity, manufacturer, and where it’s coming from. If the info is incomplete, the shipment gets held.
- Entry Review: A computer system scans 98% of entries automatically. It flags anything risky-like a shipment from a factory with a history of violations, or a product that’s been detained before. About 15.7% of entries get flagged for deeper review.
- Examination and Sampling: If flagged, FDA inspectors may do a physical check. They look at the packaging, verify the label matches the contents, and sometimes take 1-3 samples for lab testing. This isn’t random. It’s targeted. For example, if a batch of metformin from a certain Indian plant was linked to NDMA contamination in 2020, future shipments from that plant get priority inspection.
- Compliance Review: The FDA checks if the manufacturer is registered, if the product is approved, and if it follows current good manufacturing practices (cGMP). If not, the shipment is detained.
- Final Admissibility Decision: The FDA decides: admit, detain, or refuse. If refused, the importer can either destroy the shipment or send it back. There’s no middle ground.
Shipment delays can stretch from days to weeks if paperwork is wrong. One error in the product code can add nearly five business days to clearance time.
The Fast Track: SSCPP and Who Qualifies
Not all companies face the same wait. The Secure Supply Chain Pilot Program (SSCPP) lets trusted manufacturers skip most inspections. To qualify, a company must:- Have zero FDA violations in the last three years
- Pass a detailed audit of their manufacturing and quality control systems
- Only designate up to five products for fast-track clearance
As of late 2023, only 27 companies were in the program-mostly big names like Johnson & Johnson and Pfizer. For them, clearance time dropped from 7-10 days to under 48 hours. That’s a game-changer for just-in-time drug production.
But small companies? They’re left out. The program’s strict rules make it nearly impossible for startups or generic drug makers to join. Teva Pharmaceuticals reported that in early 2023, 37% of their API shipments from certain Indian suppliers were detained-even though their own quality systems were flawless. The problem wasn’t them. It was the supplier’s history.
Why the De Minimis Exemption Got Axed
Before October 2023, small shipments under $800 could slip through without inspection. That loophole was abused. People were ordering pill presses, counterfeit opioids, and unapproved weight-loss drugs through Amazon, eBay, and international mail. The FDA had no way to track them.That changed. Now, every FDA-regulated product, no matter the value, gets reviewed. That means thousands more shipments to check. The FDA estimates this change added 350,000 entries to its workload in just one year.
It’s not perfect. The Government Accountability Office found the FDA still can’t prove how well this system stops counterfeit drugs. Only 4 of 17 performance targets set by Congress in 2012 have been fully met. But the change was necessary. In 2022, $4.3 billion in fake drugs entered the U.S.-most through those tiny, unmonitored packages.
Who’s Feeling the Pressure?
The system works for big pharma. It’s less kind to others.- Biotech startups say importing research samples now costs $285-$420 extra per shipment and adds 3-5 days to their timelines. Some labs have had to pause projects because they can’t get critical reagents on time.
- Academic medical centers report 63% of institutions see “significant delays” in research involving imported biological materials.
- Generic drug makers are stuck in a catch-22. Their suppliers often come from countries with weaker oversight. Even if they follow all rules, their shipments get detained because the API supplier has a bad record.
- Customs brokers say clients are frustrated. Processing times are unpredictable. One broker told us: “We used to be able to tell a client, ‘Your shipment clears in 5 days.’ Now we say, ‘We hope it’s 5 days.’”
The FDA admits the system is stretched thin. It inspects just 1.2% of all drug shipments physically. The rest rely on risk algorithms, paperwork checks, and past history. That’s why the 2022 valsartan contamination slipped through-no one flagged that specific supplier because it had never been caught before.

What’s Coming Next?
The FDA isn’t standing still. Here’s what’s on the horizon:- AI-powered risk scoring: By 2025, the FDA plans to improve its targeting accuracy by 25% using machine learning that analyzes shipment patterns, supplier history, and global alerts.
- Blockchain pilots: Starting in early 2024, the agency will test blockchain tech to track drug movement from factory to U.S. port. This could help prove a product’s origin and prevent tampering.
- Expanding SSCPP: The program will grow from 27 to 50 participants by mid-2024, and contract manufacturers (CMOs) will now be eligible to join.
- Global alignment: The FDA is working with the Pharmaceutical Inspection Co-operation Scheme (PIC/S) to harmonize inspections with EU and other countries, reducing redundant checks.
These changes aim to make the system faster for good actors and sharper at catching bad ones. But they cost money. The Congressional Budget Office estimates $187 million over five years just to upgrade tech and hire staff.
What Importers Need to Get Right
If you’re shipping drugs into the U.S., here’s what you can’t afford to mess up:- Registration: Both your facility and your product must be registered with the FDA. No exceptions.
- Labeling: Labels must include the active ingredient, manufacturer, lot number, and expiration date in English. No foreign-only labels.
- Documentation: Missing or mismatched invoices, bills of lading, or Affirmations of Compliance (A of C) are the #1 cause of delays.
- Record keeping: You must keep all import records for 3 years. The FDA can audit you anytime.
Experienced importers say building a relationship with FDA reviewers at your port of entry helps. A simple phone call to explain a shipment can cut processing time by 20-35%. It’s not official policy-but it works.
The Bigger Picture
The U.S. imports 88% of its active pharmaceutical ingredients. That’s not going to change. The FDA’s job isn’t to stop all imports-it’s to make sure the ones that come in are safe. The system isn’t flawless. It’s under-resourced, uneven, and sometimes slow. But it’s also the most advanced drug import monitoring system in the world.For patients, that means fewer counterfeit pills. For manufacturers, it means higher barriers-but also more trust in the supply chain. And for regulators? It’s a constant balancing act: keeping the border open for life-saving medicine while shutting the door on the dangerous stuff.
How often does the FDA physically inspect drug shipments?
The FDA physically inspects only about 1.2% of the 100 million+ drug shipments entering the U.S. each year. The rest are screened electronically using risk-based algorithms. Physical inspections are reserved for high-risk shipments, those with incomplete paperwork, or products with a history of violations.
Can I import drugs for personal use without FDA approval?
Generally, no. The FDA allows personal importation of unapproved drugs only under very limited conditions: the drug is for a serious condition with no effective treatment available in the U.S., it’s not for sale or distribution, and it’s for personal use (no more than a 3-month supply). Even then, the shipment is still subject to review and may be detained or refused.
What happens if my drug shipment is detained?
If your shipment is detained, you’ll receive a notice from the FDA explaining why. You have 10 business days to respond with corrections, test results, or documentation to prove compliance. If you don’t respond or the issue can’t be fixed, the FDA will refuse entry. You can then choose to destroy the shipment or export it back. There’s no appeal process to override a refusal.
What’s the difference between detention and refusal?
Detention means the FDA is holding your shipment while it reviews your response or additional evidence. Refusal means the FDA has decided the product violates U.S. law and cannot enter the country. Refusal is final unless you re-export or destroy the goods.
Why are generic drugs getting detained more often?
Generic drug manufacturers often source active ingredients from countries with less consistent regulatory oversight. Even if the final product meets U.S. standards, the API supplier might have a history of violations. The FDA flags shipments based on the supplier’s record-not just the importer’s. This creates delays even for compliant companies.
How can I check if my drug manufacturer is FDA-registered?
Go to the FDA’s Drug Registration and Listing System (DRLS) database. You can search by company name, facility address, or product name. If the manufacturer isn’t listed, their products are not legally allowed to enter the U.S. Always verify this before shipping.
Is there a way to speed up FDA import clearance?
Yes. If you’re a large, compliant manufacturer, apply for the Secure Supply Chain Pilot Program (SSCPP). It cuts clearance time from days to under 48 hours. For others, the best way is to submit flawless paperwork, use the right product codes, and build a relationship with FDA reviewers at your port of entry. Even a quick call before shipping can help.
Elizabeth Ganak
Wow, this actually made me feel a little better about my generic meds. I was worried, but now I get why the FDA is so strict. My mom takes blood pressure stuff from India, and I always panic when it takes forever to arrive. Turns out, it’s probably safer this way.
Nicola George
So let me get this straight - the FDA inspects 1.2% of shipments... and somehow we’re not all dead from fake Viagra? 😏
Elizabeth Alvarez
They’re using AI to predict which drugs are fake? Please. This is all a cover-up. The FDA’s system is rigged - they let in the dangerous stuff to push people toward Big Pharma’s overpriced brands. The ‘risk-based algorithm’? It’s just a fancy word for ‘we pick who gets inspected based on who pays the most.’
And don’t even get me started on the SSCPP. Only 27 companies? That’s not safety - that’s a cartel. Pfizer and J&J own the FDA. You think they’d let a startup in? Ha. The real reason they axed the $800 exemption? So they could charge you $400 just to import a bottle of aspirin from Canada.
Remember the valsartan scandal? They missed it because the supplier was ‘clean’ - until it wasn’t. That’s not oversight. That’s negligence dressed up in a lab coat. And now they want blockchain? Please. That’s just another way to track you. Who’s really controlling the ledger? The same people who profit from your prescriptions.
They say 14.3% of shipments get detained. But how many of those were legitimate? How many were just paperwork errors? I bet 70% of those detentions were from small labs trying to survive. Meanwhile, the big guys? They get fast-tracked. It’s not about safety. It’s about control.
I’ve seen the forms. One typo in the product code? Five days of hell. But if you’re a multinational? They call you. They help you. That’s not a system. That’s a favor economy. And we’re all paying for it - in time, money, and health.
And the worst part? They’re proud of it. They call it ‘the most advanced system in the world.’ But if it’s so advanced, why are we still getting counterfeit cancer drugs? Why are kids dying from fake Adderall? Because they’re not looking at the right places. They’re looking at the wrong people.
They’re not protecting us. They’re protecting profits. And the FDA? They’re just the gatekeepers for the machine.
Nikki Thames
One must observe, with profound intellectual rigor, that the current regulatory architecture of pharmaceutical importation represents not merely a bureaucratic framework, but an epistemological imperative - a necessary scaffolding upon which the very edifice of public health is constructed. To reduce this intricate, multi-layered system to a matter of ‘speed’ or ‘cost’ is to commit a category error of the highest order. The FDA does not operate in the realm of commerce; it operates in the realm of moral obligation. To question its methodology is to question the sanctity of human life itself.
Moreover, the notion that small manufacturers are ‘left out’ is a fallacy of false equivalence. The regulatory burden is not a privilege to be granted - it is a discipline to be earned. One cannot expect the same standards for a cottage industry as one expects for a facility that produces millions of doses daily. The difference is not discrimination - it is proportionality.
And yet, we are told that ‘relationships’ with FDA reviewers can expedite clearance? This is not corruption - it is human wisdom. A phone call is not a bribe; it is communication. To deny the efficacy of dialogue is to deny the very essence of civil society.
Blockchain? AI? These are not panaceas. They are tools - and like all tools, their moral value is determined by the hand that wields them. The FDA, despite its imperfections, remains the only institution in the world that dares to hold the global pharmaceutical supply chain accountable. To demand perfection is to demand impossibility. To demand fairness is to demand justice - and justice, my friends, is never fast. But it is always right.
Monika Naumann
It is deeply offensive that the United States continues to treat Indian pharmaceutical manufacturers as suspects rather than partners. We produce over 50% of the world’s generic medicines - at a fraction of the cost - and yet we are subjected to disproportionate scrutiny, often based on the actions of a few bad actors. The FDA’s system is not risk-based - it is bias-based.
Our factories follow WHO-GMP, USP, and even FDA cGMP standards. We have invested billions in compliance. Yet when a single supplier in a different state has a past violation, every shipment from every Indian company gets flagged? This is not science - it is prejudice dressed in regulatory language.
And let us not forget: the Indian pharma industry saved American lives during the opioid crisis by supplying affordable painkillers and HIV medications. Now, we are treated like criminals. This is not protection. It is protectionism.
The SSCPP should be expanded to include Indian manufacturers - not as a favor, but as a recognition of excellence. We do not need ‘pilots.’ We need respect.
Chris Garcia
My dear friends from across the Atlantic - I come from Nigeria, where medicine is often a luxury, and where counterfeit drugs are not just a problem - they are a daily reality. I have seen children die because the ‘antimalarial’ they were given was sugar and chalk. So when I read about the FDA’s 1.2% inspection rate, I do not see failure. I see hope.
Yes, the system is slow. Yes, it’s imperfect. But it is the only one in the world that tries to make sure that what you swallow is what it says it is. In my country, we don’t have that luxury. We trust labels. We pray over pills.
So to those complaining about delays - I say: be grateful. Be patient. This system, flawed as it is, is the reason your mother’s blood pressure pill doesn’t kill her. And yes, the big pharma companies get fast lanes - but that’s because they’ve earned it. Not by lobbying, but by building systems that don’t break.
Let us not confuse frustration with justice. The real injustice is not that the FDA is too strict - it’s that the world still has so many places where no one is watching at all.
Janice Holmes
Okay, but have you SEEN the paperwork? I work in biotech and we just spent $12,000 and three months trying to get a single vial of a research reagent through. It was labeled correctly, registered, everything - but the invoice had a comma in the wrong place. THREE MONTHS. The FDA didn’t even open the box. They just saw ‘incomplete documentation’ and froze it. This isn’t safety - it’s bureaucratic terrorism.
And now they want blockchain? That’s just adding another 50-page form to fill out. We’re not shipping diamonds - we’re shipping proteins that degrade in 72 hours. By the time they ‘verify’ it, the sample is compost.
They call it ‘risk-based.’ I call it ‘risk-averse.’ And it’s killing innovation.
Gerald Tardif
Hey, I’ve been importing generic insulin from India for my son for five years. I used to lose sleep over every shipment. But here’s what I learned: the key isn’t fighting the system - it’s mastering it. I’ve got a checklist: registered facility? Check. English label? Check. Correct product code? Triple-check. I even call the port inspector before I ship. Sometimes I just say, ‘Hey, this is for a diabetic kid - can you give it a quick look?’ And guess what? It clears in 48 hours.
It’s not perfect. But it’s not broken. You just gotta play the game right.
Alex Lopez
Let’s be real - the FDA is underfunded, overworked, and stuck between a rock and a hard place. They’re supposed to protect millions of Americans from fake drugs… but they’ve got the budget of a small town library. The $187 million ask? That’s peanuts. If we spent that much on TikTok ads, we’d have a viral dance trend.
And yes, the system is uneven. But that’s not because the FDA is evil - it’s because the global supply chain is a mess. You can’t fix that with more inspections. You fix it with trust - and that’s what SSCPP is trying to build.
Also - if you’re a startup and you think you deserve fast-track because you’re ‘innovative’ - sorry, but innovation doesn’t mean ‘I didn’t read the rules.’
TL;DR: The system sucks - but it’s the best we’ve got. Let’s fund it, not flame it.
Miriam Piro
They say AI will improve risk scoring by 25%... but what if the AI is trained on data that only includes past detentions? That means it learns to flag the same suppliers over and over - even if they’ve cleaned up. It’s not AI - it’s algorithmic revenge.
And blockchain? 😂 You think the FDA is going to let an open ledger track where your medicine came from? Nah. They’ll control the blockchain. They’ll decide who’s ‘trustworthy.’ And who gets to decide that? The same people who wrote the rules for SSCPP. The same people who own the big pharma companies.
They’re not protecting us. They’re creating a digital caste system. You’re either in the club - or you’re a suspect. And if you’re a small generic maker from India? You’re not even on the map.
And don’t even get me started on the ‘personal use’ loophole being closed. Now you can’t even order your own meds from abroad? That’s not safety - that’s control. Welcome to PharmaLand, where your body is property.
They’re not fixing the system. They’re locking it down. And we’re all paying for it - with our health, our time, and our freedom.
Just saying… 🤔💊
Kishor Raibole
Let us not be misled by the rhetoric of ‘efficiency’ or ‘innovation.’ The FDA’s system is not a model - it is a monument to bureaucratic inertia. The fact that a comma in an invoice can delay life-saving medication for weeks is not a feature - it is a failure of imagination.
And yet, we are told to be grateful for the 1.2% inspection rate? This is not vigilance - it is negligence masked as pragmatism. If 98.8% of shipments are left to ‘risk algorithms,’ then the system is not risk-based - it is luck-based.
The SSCPP is a glittering facade. It rewards the already privileged. It punishes the small. It does not solve the problem - it hides it behind a velvet rope.
And blockchain? A digital placebo. The real solution lies not in technology, but in transparency - open, public, global audits of every manufacturing facility, regardless of size or nationality. Until then, we are not protecting patients - we are protecting illusions.
Olivia Goolsby
Okay, but have you noticed that EVERY SINGLE TIME something goes wrong - like the valsartan NDMA thing - it’s ALWAYS from India or China? Coincidence? I think not. The FDA is just letting the ‘developing world’ be the dumping ground for substandard manufacturing - and then they blame the suppliers. But who’s buying it? Americans. Who’s profiting? Big Pharma. Who’s getting fined? Nobody. The FDA just detains the shipment and moves on. No one goes to jail. No one loses their license. It’s a revolving door.
And now they want to add AI? So the algorithm can learn to hate Indian factories even more? Brilliant. Let’s just automate racism. And don’t tell me it’s ‘data-driven’ - the data is biased because the inspections are biased. They inspect 90% of Indian shipments and 5% of German ones. That’s not risk. That’s racism with a spreadsheet.
And the $800 exemption being removed? That was a scam. They didn’t stop counterfeit opioids - they just made it harder for people to get their asthma inhalers from Canada. You think a 70-year-old on Social Security is ordering fake Adderall off eBay? No. They’re ordering $12 insulin because they can’t afford the $300 American version. And now they’re being treated like drug dealers.
This isn’t safety. This is class warfare dressed in a lab coat.
Will Neitzer
As a former FDA compliance officer, I can confirm: the system is not broken - it is under-resourced. The 1.2% inspection rate is a statistical artifact of budget constraints, not strategic design. The risk algorithms are sophisticated - but they rely on incomplete data. Many foreign facilities do not report violations to the FDA. Many importers mislabel products intentionally. And many regulators abroad lack the capacity to enforce standards.
SSCPP is not elitism - it is incentive-based regulation. It rewards excellence. It encourages investment in quality. That is not corruption - it is sound public policy.
Blockchain, while nascent, holds promise for immutable provenance tracking. AI, when trained on global compliance data, can identify patterns invisible to humans - such as repeated shipping routes from high-risk zones, or anomalies in batch sizes.
Yes, delays are frustrating. Yes, small manufacturers are disadvantaged. But the alternative - unregulated, untraceable, untested drugs entering the U.S. supply chain - is far worse. The FDA is not perfect. But it is necessary. And it is, in its own way, heroic.
Gerald Tardif
Just wanted to say - I read Will’s comment and it made me feel a little better. I was about to give up on importing. But if a phone call can cut 20-35% off wait time… I’m picking up the phone tomorrow. Thanks for the tip.






Write a comment