When you’re managing a chronic autoimmune disease like rheumatoid arthritis, psoriasis, or severe eczema, the idea of swapping daily injections for a simple pill can feel like a game-changer. That’s exactly what JAK inhibitors offer. These oral drugs, also known as jakinibs, work inside your cells to block the signals that drive inflammation - without targeting a single protein like older biologics do. Since tofacitinib became the first FDA-approved JAK inhibitor in 2012, the class has exploded, with six drugs now on the market and more coming. But behind the convenience and rapid relief lies a complex safety profile that demands careful monitoring.
How JAK Inhibitors Work
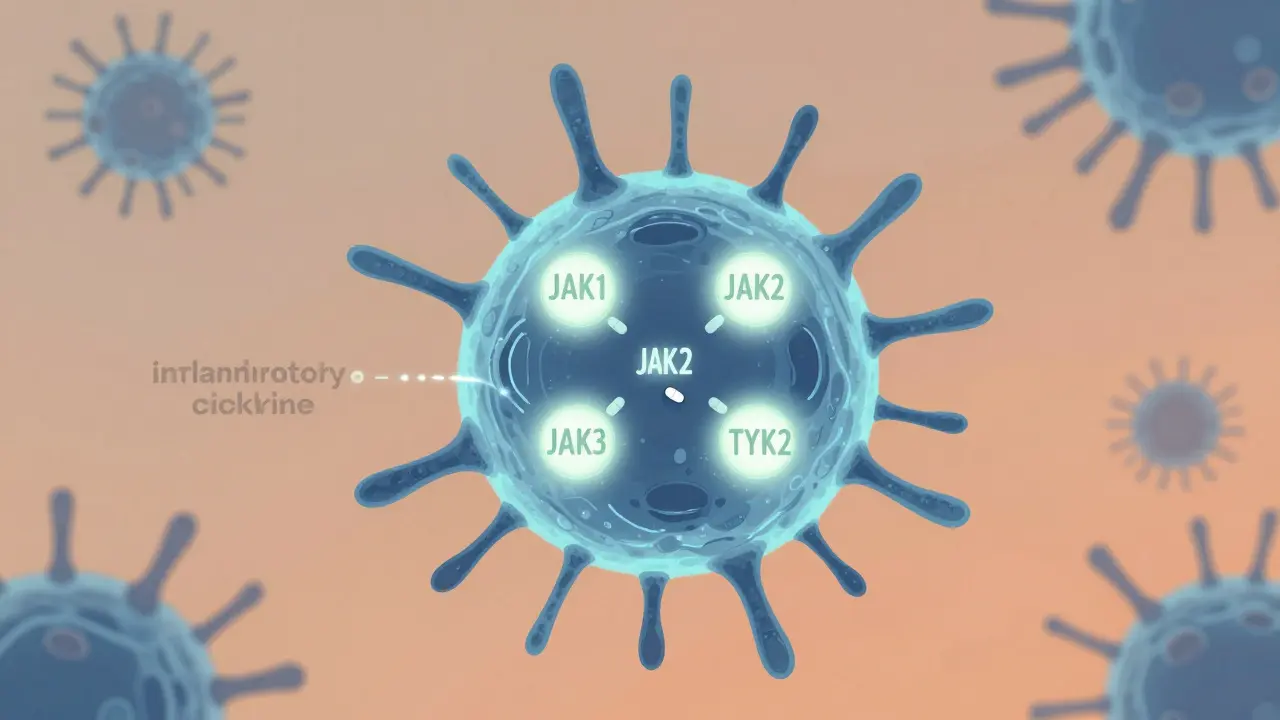
JAK inhibitors don’t just calm inflammation - they cut it off at the source. Inside your immune cells, proteins called Janus kinases (JAK1, JAK2, JAK3, and TYK2) act like switches that turn on inflammation when cytokines bind to receptors. When you take a JAK inhibitor, it slips into the active site of these enzymes and blocks them from sending the signal that triggers the immune system to attack your own tissues. Unlike biologics that target one cytokine at a time - like TNF or IL-17 - JAK inhibitors shut down multiple pathways at once. That’s why one drug can help with both joint pain and skin flares.
Some drugs in this class are more selective than others. Upadacitinib, for example, is designed to favor JAK1 over JAK2, reducing side effects linked to JAK2 inhibition like anemia or low platelets. Abrocitinib zeroes in on JAK1 with high precision, making it effective for eczema with fewer blood-related issues. Ritlecitinib takes a different approach: it binds permanently to JAK3, blocking it in a way that’s harder for the body to bypass. Even newer agents like deuruxolitinib and brepocitinib are being built to target TYK2 specifically, aiming to keep the immune system’s defensive functions intact while silencing the inflammatory noise.
Why They’re Changing the Game
For patients who’ve tried multiple biologics without success, JAK inhibitors often deliver results faster. Many people notice symptom improvement within two to four weeks - compared to eight to twelve weeks with TNF blockers. In clinical trials, up to 71% of rheumatoid arthritis patients on upadacitinib saw at least a 20% improvement in joint swelling and pain within 12 weeks. For those with alopecia areata, deuruxolitinib helped over half of patients regrow more than half their scalp hair in six months.
The convenience factor is huge. No more freezing vials, needle phobia, or clinic visits for infusions. A daily pill fits into life more easily. A 2023 survey of over 1,200 patients found that 92% preferred oral treatment over injections. On forums like Reddit and HealthUnlocked, people share stories of going from being unable to hold a cup to playing with their kids again - all within weeks.
The Hidden Risks
But there’s a cost. In January 2022, the FDA added a black box warning - the strongest possible - to all JAK inhibitors. The warning highlights four major risks: serious infections, cancer, major heart events like heart attack or stroke, and blood clots. These aren’t theoretical. The ORAL Surveillance study, which followed over 4,000 rheumatoid arthritis patients over eight years, found that those on tofacitinib had a 31% higher risk of major cardiovascular events and a 49% higher risk of cancer compared to those on TNF inhibitors.
Herpes zoster (shingles) is one of the most common side effects. Around 23% of users report reactivation, compared to just 3% on biologics. That’s why many doctors now prescribe antivirals like valacyclovir as a preventive measure, especially for patients over 50 or with a history of shingles. Lipid levels also tend to rise - LDL cholesterol can jump by 20-30 mg/dL in the first few months. That’s not just a lab number; it’s a signal that your cardiovascular risk is increasing.
And it’s not just about the drug. It’s about who’s taking it. The European League Against Rheumatism (EULAR) and American College of Rheumatology (ACR) now agree: JAK inhibitors should not be used in patients over 65 with heart disease, smokers, or anyone with a past cancer diagnosis. In Australia, rheumatologists are even more cautious. Many wait until after two biologics have failed before prescribing them.

What You Must Monitor
If you’re on a JAK inhibitor, regular blood tests aren’t optional - they’re lifesaving. The ACR recommends baseline tests before you start, then every three months for the first year, and every six months after that. Here’s what your doctor is watching:
- Complete blood count (CBC): Lymphocyte count below 500 cells/μL means stop the drug. Low hemoglobin (below 8 g/dL) or platelets can signal bone marrow suppression.
- Liver enzymes (ALT, AST): Levels over three times the upper limit require immediate evaluation.
- Lipid panel: LDL above 190 mg/dL triggers a statin prescription. Many patients need cholesterol meds just to stay safe.
- Tuberculosis screening: A chest X-ray and PPD or interferon-gamma release assay are required before starting.
Before you even take your first pill, your doctor should check if you’ve had chickenpox or the vaccine. If not, you need the varicella vaccine at least four weeks before starting. Yet, a 2023 EULAR survey found that nearly 70% of clinics skip this step because they’re in a rush to treat.
Who Should Avoid Them
Not everyone is a candidate. If you have:
- A history of heart attack, stroke, or blood clots
- Active or recent cancer (within the last five years)
- Uncontrolled high blood pressure or diabetes
- Chronic lung disease or recurrent infections
- Are over 65 with any cardiovascular risk factors
…then JAK inhibitors are not the right choice. Even if your symptoms are bad, the long-term risks outweigh the benefits. There are safer alternatives - newer biologics like IL-23 or IL-17 inhibitors - that offer similar efficacy without the same safety red flags.
The Future: Better, Safer Drugs
The next wave of JAK inhibitors is already in the pipeline. Brepocitinib, a dual TYK2/JAK1 inhibitor, is in phase 3 trials and could be approved by late 2025. It’s designed to avoid JAK2 entirely, which could eliminate the anemia and cholesterol spikes seen with older drugs. Ritlecitinib’s covalent binding mechanism might allow for lower doses and less frequent dosing. And researchers are exploring once-weekly formulations to improve adherence.
But the biggest shift isn’t in the drugs - it’s in the mindset. Doctors are moving away from seeing JAK inhibitors as “better biologics.” Instead, they’re treating them like high-risk medications that require strict patient selection and lifelong monitoring. The goal isn’t just to control symptoms - it’s to keep you alive and healthy for decades.
Real Stories, Real Trade-Offs
One patient in Sydney, 58, with rheumatoid arthritis and psoriasis, started baricitinib after three biologics failed. Within six weeks, her joint pain dropped from 18 swollen joints to two. Her skin cleared. But her LDL jumped from 110 to 178. She now takes a statin, gets blood work every three months, and takes daily antivirals. “It’s not perfect,” she says. “But I can hold my granddaughter without pain. That’s worth the extra tests.”
Another, 42, with severe eczema, took abrocitinib and saw her skin clear in ten days. Two months later, she got shingles. “I thought I was done with flares forever,” she says. “Then I got it twice. Now I’m scared to stop - but also scared to keep going.”
There’s no one-size-fits-all answer. JAK inhibitors are powerful tools - but they’re not magic pills. They’re high-stakes interventions that demand partnership between patient and doctor.
Are JAK inhibitors better than biologics?
It depends. JAK inhibitors are easier to take (oral vs. injection), work faster, and can treat multiple conditions at once - like arthritis and eczema together. But biologics have a longer safety track record. For younger, healthy patients without heart disease or cancer history, JAK inhibitors may be a good option. For older patients or those with cardiovascular risks, biologics are still the safer first choice.
How long do I need to take JAK inhibitors?
Most people stay on them long-term, as stopping often leads to flare-ups. But if you develop serious side effects - like cancer, a blood clot, or uncontrolled infections - your doctor will stop the drug immediately. Some patients switch to newer biologics if they become available and safer. There’s no fixed timeline - it’s based on how you respond and how your body handles the drug over time.
Can I drink alcohol while on JAK inhibitors?
Moderate alcohol is usually okay, but heavy drinking increases liver stress - and JAK inhibitors already affect liver enzymes. Alcohol also weakens your immune system, which raises infection risk. Most doctors recommend limiting alcohol to no more than one drink per day, if at all. Always check with your rheumatologist or dermatologist, especially if your liver numbers are rising.
Do JAK inhibitors cause weight gain?
Weight gain isn’t a direct side effect of JAK inhibitors. But some people gain weight because their inflammation is under control - they feel better, eat more, and move less. Others gain due to steroid use before starting JAK inhibitors, or because of other medications like prednisone. If you notice unexplained weight gain, talk to your doctor - it could signal fluid retention or metabolic changes linked to rising cholesterol or insulin resistance.
What happens if I miss a dose?
If you miss one dose, take it as soon as you remember - unless it’s almost time for your next dose. Then skip the missed one. Don’t double up. Missing doses can cause flare-ups, especially in the first few months. But occasional missed doses won’t trigger a crisis. The bigger risk is stopping completely without medical advice - that’s when symptoms come back hard and fast.
Next Steps: What to Do Now
If you’re considering a JAK inhibitor:
- Get a full health review - including heart, lung, and cancer history.
- Ask about your cholesterol and blood counts - baseline numbers matter.
- Confirm you’ve had chickenpox or the vaccine. If not, schedule the shot now.
- Discuss alternatives: newer biologics might be safer for you.
- Set up a monitoring schedule before you start - don’t wait for symptoms to change.
If you’re already on one:
- Stick to your blood test schedule - no skipping.
- Report any new fever, rash, chest pain, or shortness of breath immediately.
- Ask your doctor about antiviral prophylaxis if you’re over 50 or had shingles before.
- Don’t stop the drug on your own - even if you feel fine.
JAK inhibitors changed how we treat autoimmune disease. But they’re not a shortcut. They’re a responsibility - one that requires awareness, discipline, and a strong partnership with your care team. The goal isn’t just to feel better today. It’s to stay healthy for the long haul.
Jon Paramore
JAK inhibitors are potent but non-selective kinase blockers-off-target inhibition of JAK2 drives anemia and thrombocytopenia, while JAK1 blockade mediates both efficacy and increased herpes zoster risk. The black box warning is justified: HR 1.31 for MACE, HR 1.49 for malignancy in ORAL Surveillance. Newer agents like brepocitinib (TYK2/JAK1 selective) may decouple efficacy from hematologic toxicity. Still, lipid elevations are class-wide and require statin co-therapy in ~40% of patients.
Jackie Be
I took abrocitinib for 8 months and my skin was flawless like i was reborn but then i got shingles twice and now im scared to even think about stopping because the eczema comes back worse than ever like its angry at me
Cara C
That story about the shingles really hits hard. I know so many people who’ve been through this-feeling like they’re stuck between relief and fear. It’s not just about the drug, it’s about the emotional toll of never knowing if the next rash or fever is the one that means you have to stop.
Swapneel Mehta
As someone from India with a family history of TB, I’m glad the screening requirement is mentioned. Many clinics here skip baseline PPD tests because they’re rushed. But latent TB reactivation under JAK inhibitors is no joke-seen it firsthand. Always get that chest X-ray, even if your doctor says it’s ‘probably fine’.
Michael Ochieng
Just want to say thanks for laying this out so clearly. I’m a nurse in a rheum clinic and we see this daily-patients excited about the pill, then scared after the black box warning. The monitoring schedule you listed? That’s exactly what we enforce now. Bloodwork every 3 months, no exceptions. It’s not paranoia-it’s prevention.
Dan Adkins
While the clinical data is robust, the real-world application remains fraught with systemic neglect. The EULAR survey indicating 70% of clinics omit varicella vaccination prior to initiation is not merely an oversight-it is a breach of the duty of care. JAK inhibitors are not ‘convenient biologics’; they are immunosuppressive agents requiring pre-emptive, protocol-driven risk mitigation. The absence of standardized pre-screening protocols across healthcare systems constitutes a latent public health liability.
Christina Weber
People act like JAK inhibitors are a miracle cure, but they ignore the fact that you’re trading long-term safety for short-term comfort. If you’re over 50, have any cardiovascular risk, or even smoked once in your life-you shouldn’t be on this. And yet, doctors prescribe them like they’re Advil. It’s irresponsible. Your cholesterol isn’t just a number-it’s a ticking clock. And if you miss a blood test? You’re gambling with your life.
John Hay
My dad’s on baricitinib. He’s 62, had a stent in 2020, and his LDL jumped to 210. They put him on a statin, started antivirals, and now he gets labs every 3 months. He says he’d rather do that than be in pain. I don’t think people realize how much work this takes. It’s not just popping a pill. It’s a whole lifestyle shift.
Meina Taiwo
For Nigerian patients: access to labs is a huge barrier. Many can’t afford monthly blood tests. If you’re considering JAK inhibitors, talk to your doctor about alternatives-some biologics are cheaper long-term if you factor in monitoring costs. Don’t let convenience override sustainability.
Jerry Peterson
My cousin’s on ritlecitinib for alopecia. She’s 28, no other health issues. Hair’s growing back like crazy. No shingles, no cholesterol spike. She’s lucky-her doctor was super careful about screening. But she still gets labs every 6 months. No skipping. She says it’s the price of getting her confidence back.
Grace Rehman
So we’ve got a drug that lets you hold your granddaughter again… but also increases your risk of cancer and stroke. And we call this progress? Maybe we’re just really good at convincing ourselves that risk is worth it when we’re in pain. But let’s not pretend it’s not a trade-off. We’re not curing disease-we’re buying time with a credit card made of blood tests and statins.
Orlando Marquez Jr
The future of JAK inhibitors lies not in further molecular refinement, but in implementation science. The disconnect between guideline adherence and clinical practice remains profound. Without mandatory electronic health record alerts for baseline screening, lipid monitoring, and antiviral prophylaxis, even the most elegant pharmacology will fail at the bedside. System-level interventions are non-negotiable.






Write a comment