Fournier's Gangrene Symptom Checker
Check Your Symptoms
Select any symptoms you're experiencing. If you have any symptoms, seek emergency medical attention immediately.
More than 12 million Americans take SGLT2 inhibitorsa class of medications used to treat type 2 diabetes. These drugs help control blood sugar by removing excess glucose through urine. But a rare but deadly complication called Fournier's gangrene can occur. Knowing the warning signs and acting fast is critical.
What Are SGLT2 Inhibitors?
SGLT2 inhibitors are a type of diabetes medication first approved by the U.S. Food and Drug Administration (FDA) in 2013. They work by blocking sodium-glucose cotransporter-2 in the kidneys. This prevents glucose reabsorption, leading to more sugar being excreted in urine. Common brand names include Invokana (canagliflozin), Farxiga (dapagliflozin), Jardiance (empagliflozin), and Steglatro (ertugliflozin). These medications are prescribed for type 2 diabetes to improve blood sugar control and reduce the risk of heart and kidney complications. Unlike older diabetes drugs, SGLT2 inhibitors lower blood sugar without causing hypoglycemia (low blood sugar) because they work independently of insulin. They also help with weight loss and blood pressure control, making them a valuable option for many patients.
Understanding Fournier's Gangrene
Fournier's gangrene is a severe bacterial infection that destroys tissue in the genital and perineal areas. It's a form of necrotizing fasciitis-often called "flesh-eating disease"-but specifically affects the groin region. The infection spreads rapidly and can be fatal if not treated immediately. While it's extremely rare, the risk increases for people taking SGLT2 inhibitors. The FDA issued a boxed warning in August 2018 after 12 cases were reported between 2013 and 2018. This rare complication has a mortality rate of 4-8%, making early detection vital.
The mechanism behind this risk isn't fully understood, but researchers believe the glucose in urine from SGLT2 inhibitors creates a breeding ground for bacteria. This, combined with potential immune system changes, may trigger the infection. Most cases occur in people with diabetes, especially those with poor blood sugar control. However, even well-managed diabetes patients can develop this condition.
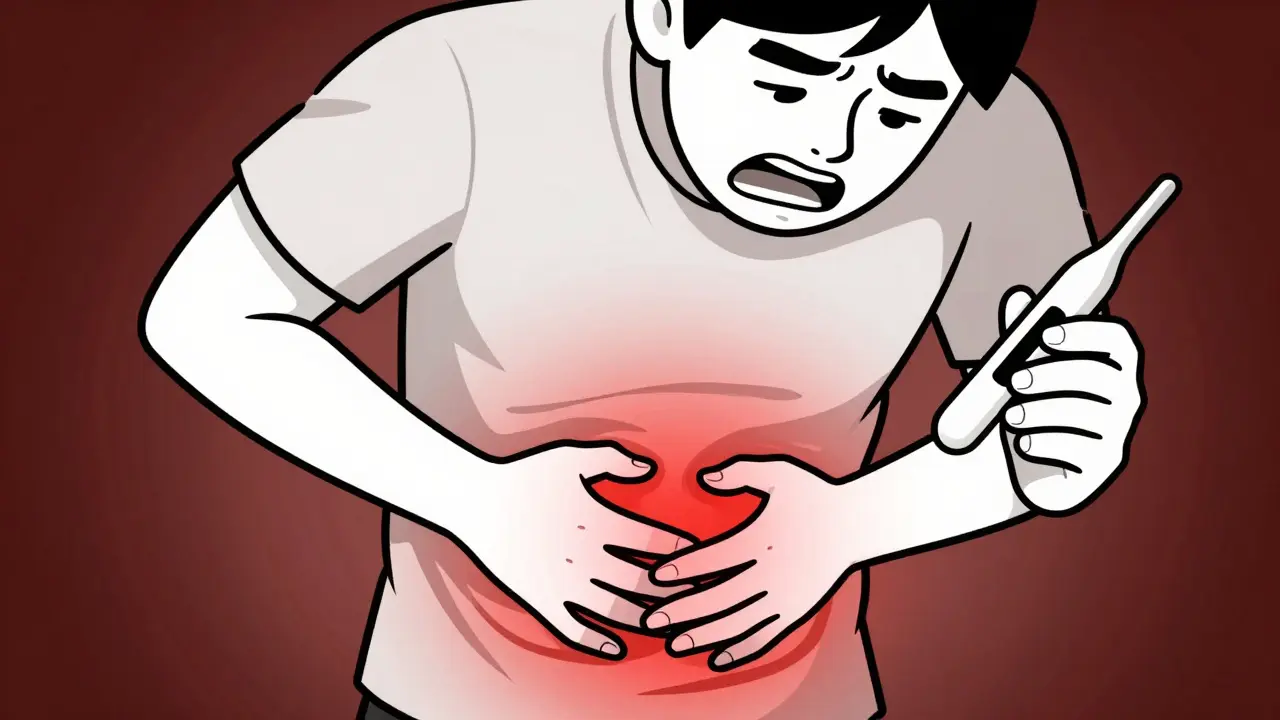
Early Warning Signs You Must Know
Symptoms of Fournier's gangrene develop quickly, often within hours. Watch for:
- Severe pain or tenderness in the genital or anal area (often worse than expected from the visible symptoms)
- Redness, swelling, or warmth in the skin around the genitals
- Fever or chills (usually above 100.4°F)
- Malaise (general feeling of being unwell)
- Skin discoloration (black or purple patches indicating dead tissue)
- A foul odor from the affected area
- Blisters or ulcers that worsen rapidly
These symptoms can escalate rapidly. If you experience any of these while taking an SGLT2 inhibitor, seek emergency care immediately. Don't wait-this is not something to monitor at home. Early diagnosis is critical for survival.
Immediate Actions to Take
If you notice warning signs of Fournier's gangrene:
- Stop taking your SGLT2 inhibitor right away.
- Call 911 or go to the nearest emergency room immediately. Tell healthcare providers you're taking an SGLT2 inhibitor and suspect Fournier's gangrene.
- Be prepared for surgery: treatment typically involves removing dead tissue (debridement), which may require multiple procedures. Intravenous antibiotics are started immediately to fight the infection.
Every hour counts-delaying treatment increases the risk of death by about 9% per hour. Most patients need intensive care and long-term recovery. Some require reconstructive surgery later. Do not delay-this is a medical emergency.

How Rare Is This Risk?
While the thought of a "flesh-eating" infection sounds scary, the actual risk is very low. Studies show approximately 1 additional case per 10,000 men treated with SGLT2 inhibitors. The FDA's initial review found 12 cases over five years, but this number has likely increased slightly since then. The European Medicines Agency reports about 1.9 cases per 100,000 patient-years. For context, diabetes itself increases the risk of infections due to weakened immunity. However, the combination of SGLT2 inhibitors and diabetes creates a unique risk profile. Despite the rarity, the severity makes awareness essential.
In the United Kingdom, the Medicines and Healthcare products Regulatory Agency (MHRA) recorded 6 cases of Fournier's gangrene linked to SGLT2 inhibitors (4 in men and 2 in women) up to January 2019. This was based on an estimated 548,565 patient-years of treatment. Similar data exists in the U.S. and Europe, confirming the risk is real but rare.
| Drug Name | Brand Name | FG Risk Status |
|---|---|---|
| Canagliflozin | Invokana | Confirmed cases |
| Dapagliflozin | Farxiga | Confirmed cases |
| Empagliflozin | Jardiance | Confirmed cases |
| Ertugliflozin | Steglatro | Insufficient data |
When to Talk to Your Doctor
Most people taking SGLT2 inhibitors never develop Fournier's gangrene. But certain factors increase risk:
- Poorly controlled diabetes (HbA1c above 9%)
- History of genital infections (like yeast infections)
- Weakened immune system (from conditions like HIV or medications)
- Obesity or other conditions affecting circulation
Discuss these risks with your doctor before starting SGLT2 inhibitors. They'll help weigh benefits against potential side effects. If you're already on these medications, keep regular check-ups. Report any unusual symptoms immediately. Your doctor might adjust your treatment plan based on your health history.
The American Diabetes Association continues to recommend SGLT2 inhibitors as part of standard care for type 2 diabetes. They emphasize that the benefits-like reduced heart failure hospitalizations and kidney disease progression-outweigh the risks for most patients when used appropriately. But they also stress the importance of patient education about this rare side effect.
How common is Fournier's gangrene with SGLT2 inhibitors?
Extremely rare. Studies estimate about 1 additional case per 10,000 men treated. For context, the FDA identified 12 cases between 2013 and 2018 across all SGLT2 inhibitors. The European Medicines Agency reports approximately 1.9 cases per 100,000 patient-years. While the risk is low, the severity makes early detection crucial.
Can women get Fournier's gangrene from SGLT2 inhibitors?
Yes. While Fournier's gangrene typically affects men (about 90% of cases), about one-third of reported cases linked to SGLT2 inhibitors occurred in women. This means all users of these medications should be aware of symptoms regardless of gender.
Should I stop taking my SGLT2 inhibitor because of this risk?
No, unless you experience symptoms. The benefits of SGLT2 inhibitors for most people with type 2 diabetes outweigh the risk. These drugs reduce heart failure hospitalizations and kidney disease progression. Only stop the medication if you notice warning signs of Fournier's gangrene and seek immediate medical help. Never discontinue diabetes medication without consulting your doctor.
What happens if I delay treatment for Fournier's gangrene?
Delaying treatment significantly increases mortality risk. Each hour of delay raises the chance of death by about 9%. Immediate surgery and antibiotics are required. Patients who wait more than 24 hours often face severe complications or death. If you suspect symptoms, act immediately-don't wait to see if it gets better.
Are all SGLT2 inhibitors equally risky for Fournier's gangrene?
All currently approved SGLT2 inhibitors carry this risk. Canagliflozin, dapagliflozin, and empagliflozin have confirmed cases linked to them. Ertugliflozin (Steglatro) has less data, but regulatory agencies treat it as part of the class risk. This is why all SGLT2 inhibitor labels include warnings about Fournier's gangrene.
Brendan Ferguson
I've been on Invokana for three years. Never had issues, but I'll keep an eye out for symptoms. Better safe than sorry.
jan civil
Severe genital pain? ER immediately. No waiting.
Kate Gile
Early detection is key. If you notice any signs, act fast. Your health is worth it.
Matthew Morales
I've been on Jardiance for a few years now and never had any issues, but after reading this post I'm definitely more aware. I didn't know that Fourniers gangrene was linked to these meds. It's scary to think about, but I guess it's important to know. The symptoms they listed like severe pain in the genital area, fever, or skin discoloration-those are things I'd want to catch early. I'll keep an eye out for those signs. Also, I'm not saying to stop taking your meds, but if you notice anything weird, go to the ER right away. I'm not a doctor, but I think it's better to be safe. I've been on these meds for years without problems, but it's good to know what to look for. The fact that it's rare but serious means you shouldn't panic, but you should stay informed. Maybe talk to your doctor if you're worried. They can help you weigh the risks. I'm glad the FDA issued a warning so people know. This is one of those things where awareness can make all the difference. I'll definitely mention this to my friends who are on similar meds. It's important to spread the word. Just don't freak out, but stay vigilant. That's my two cents. 😅
Diana Phe
Big Pharma knows this but hides it. They don't care about patients. Always be suspicious.
Tehya Wilson
Fournier's gangrene rare but serious. Act immediately if symptoms present.
Johanna Pan
SGLT2 inhibitors help many people. Just need to know the rare risks. Check with your doctor regularly.
Jenna Elliott
Big Pharma hiding this. No trust. Always suspicious.
Elliot Alejo
SGLT2 inhibitors are safe for most. Know the signs and act fast. That's all.
lance black
Symptoms need ER immediately.
Laissa Peixoto
While the risk is low, awareness saves lives. It's about balancing benefits and risks thoughtfully.




Write a comment